Microalgae growth and oxygen production on different textile fabrics
DOI:
https://doi.org/10.25367/cdatp.2022.3.p9-16Keywords:
green microalgae, knitted fabrics, Tencel, cotton, linen, oxygen production, Clark electrodeAbstract
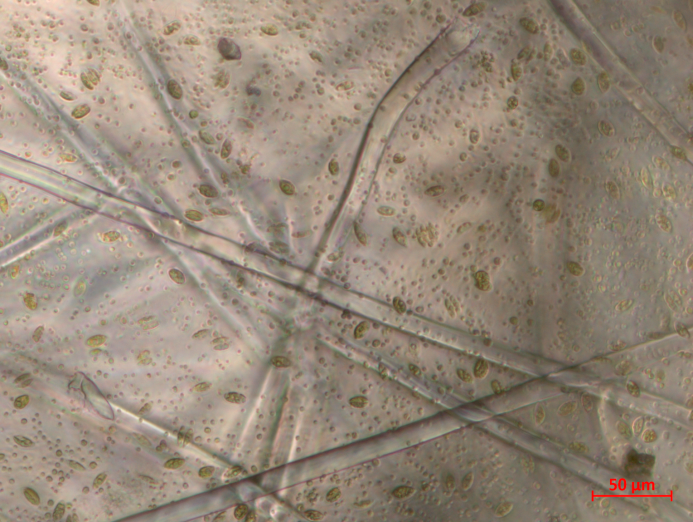
Microalgae can be used for diverse applications in research and industry. Several microalgae grow adhering to surfaces that are usually two-dimensional. A third dimension could increase the amount of microalgae adhering to a given area and can be offered by textile fabrics. Here we report on the microalgae Chlorella vulgaris and Scenedesmus spec. growing on different knitted fabrics under defined light and under office light conditions. Our results show a significant influence of illumination on both algal species and a smaller impact of the chosen medium, while all knitted fabrics under examination were found well suited as substrates. The numbers of alga cells per petri dish were higher on textile fabrics than in pure water or medium by a factor of ~ 4–20, respectively.
References
González-Fernández, C.; Sialve, B; Bernet, N.; Steyer, J. P. Impact of microalgae characteristics on their conversion to biofuel. Part I: focus on cultivation and biofuel production. Biofuel Bioprod. Biorefin. 2012, 6, 105-113. DOI 10.1002/bbb.338.
Homburg, S. V.; Kruse, O.; Patel, A. V. Growth and photosynthetic activity of Chlamydomonas reinhardtii entrapped in lens-shaped silica hydrogels. J. Biotechnol. 2019, 302, 58-66. DOI 10.1016/j.jbiotec.2019.06.009.
Homburg, S. V.; Venkanna, D.; Kraushaar, K.; Kruse, O.; Kroke, E.; Patel, A. V. Entrapment and growth of Chlamydomonas reinhardtii in biocompatible silica hydrogels. Coll. Surf. B Biointerfaces 2019, 173, 233-241. DOI 10.1016/j.colsurfb.2018.09.075.
Fradique, M.; Batista, A. P.; Nunes, M. C.; Gouveia, L.; Bandarra, N. M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656-1664. DOI 10.1002/jsfa.3999.
Becker, W. Microalgae in human and animal nutrition. In Handbook of microalgal culture; Richmond, A., Ed.; Blackwell, Oxford, 2004; pp 312-351.
Borowitzka, M. A. Commercial production of microalgae: ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313-321. DOI 10.1016/S0168-1656(99)00083-8.
Lee, Y. K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307-315. DOI 10.1023/A:1017560006941.
Ozkan, A.; Kinney, K.; Katz, L.; Berberoglu, H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012, 114, 542-548. DOI 10.1016/j.biortech.2012.03.055.
Sekar, R.; Venugopalan, V.; Satpathy, K.; Nair, K. V. K.; Rao, V. N. R. Laboratory studies on adhesion of microalgae to hard substrates. Hydrobiologia 2004, 512, 109-116. DOI 10.1023/B:HYDR.0000020315.40349.38.
Johnson, M. B.; Wen, Z. Y. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010, 85, 525–534. DOI 10.1007/s00253-009-2133-2.
Ozkan, A.; Berberoglu, H. Adhesion of algal cells to surfaces. Biofouling 2013, 29, 469-482. DOI 10.1080/08927014.2013.782397.
Melo, M.; Fernandes, S.; Caetano, N.; Borges, M. T. Chlorella vulgaris (SAG 211-12) biofilm formation capacity and proposal of a rotating flat plate photobioreactor for more sustainable biomass production. J. Appl. Phycol. 2018, 30, 887-899. DOI 10.1007/s10811-017-1290-4
Gao, F.; Yang, Z.-H.; Li, C; Zeg, G.-M.; Ma, D.-H.; Zhou, L. A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour. Technol. 2015, 179, 8-12. DOI 10.1016/j.biortech.2014.11.108.
Shen, Y.; Yang, T.; Zhu, W.; Zhao, Y. Wastewater treatment and biofuel production through attached culture of Chlorella vulgaris in a porous substratum biofilm reactor. J. Appl. Phycol. 2017, 29, 833-841. DOI 10.1007/s10811-016-0981-6.
Shen, Y.; Zhu, W.; Chen, C.; Nie, Y.; Lin, X. Biofilm formation in attached microalgal reactors. Bioproc. Biosyst. Eng. 2016, 39, 1281-1288. DOI 10.1007/s00449-016-1606-9.
Großerhode, C.; Wehlage, D.; Grothe, T.; Grimmelsmann, N.; Fuchs, S.; Hartmann, J.; Mazur P.; Reschke, V.; Siemens, H.; Rattenholl, A.; Homburg, S. V.; Ehrmann, A.) Investigation of microalgae growth on electrospun nanofiber mats. AIMS Bioeng. 2017, 4, 376-385. DOI 10.3934/bioeng.2017.3.376.
Sebök, S.; Brockhagen, B.; Storck, J. L.; Post, I. B.; Bache, T.; Korchev, R.; Böttjer, R.; Grothe, T.; Ehrmann, A. Growth of marine macroalgae Ectocarpus sp. on various textile substrates. Environmental Technology 2020, online first. DOI 10.1080/09593330.2020.1829086.
Brockhagen, B.; Storck, J. L.; Grothe, T.; Böttjer, R.; Ehrmann, A. Improved growth and harvesting of microalgae Chlorella vulgaris on textile fabrics as 2.5D substrates. AIMS Bioeng. 2020, 8, 16-24. DOI 10.3934/bioeng.2021003.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Ewin Tanzli, Bennet Brockhagen, Inken Blanka Post, Thorsten Bache, Khorolsuren Tuvshinbayar, Sarah Vanessa Homburg, Andrea Ehrmann

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





